Suttons Creek partners with pharmaceutical companies, providing services including expert consulting, embedded execution, training and guidance for the development of combination products. Our services create success in global regulatory affairs, quality systems, device engineering, commercial launch and digital support platforms throughout the entire product lifecycle.
We can customize our services to meet your immediate needs, your short-term initiatives, or your long-term strategic goals. For those clients who require smaller steps, we offer on-call, hourly consulting contracts to guide and execute as needed and as budgets allow.
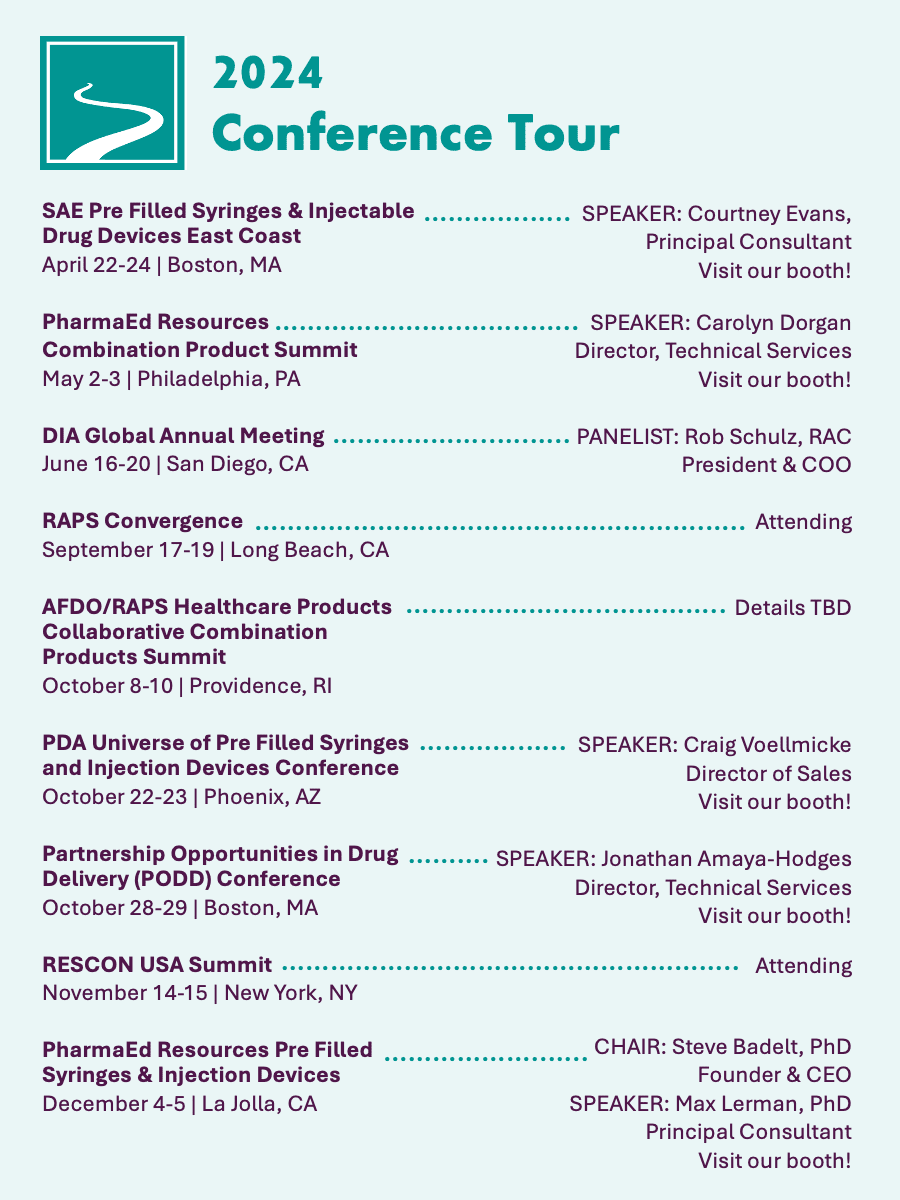
Join us at key combination product events this year…
Our team will be heading to the below events and hope to see you while we are there. If you are interested in meeting with our team members, you can also always complete our contact form, call us, or click the button below to connect.
If you are a trade event organizer and have an interest in any of our experts speaking at your event, please email us.
Links to the conferences we recommend for Combination Product developers:
SAE Media Group Pre Filled Syringes & Injectable Drug Devices East Coast
PharmaEd Resources Combination Product Summit
AFDO/RAPS Healthcare Products Collaborative Combination Products Summit
PDA Universe of Pre Filled Syringes and Injection Devices Conference
Partnership Opportunities in Drug Delivery (PODD) Conference

FORMER FDA REVIEWER COURTNEY EVANS WILL GUIDE YOU THROUGH NAVIGATING THE CONNECTED DEVICES LANDSCAPE
Are you developing a connected combination product or considering entering this space? Suttons Creek Principal Consultant Courtney Evans will walk you through the current landscape of digital drug delivery systems and best practices for developing and gaining regulatory approval for connected software devices at SAE Media Group’s Pre Filled Syringes and Injectable Drug Devices East Coast in Boston on April 24th. Join them and gain insight from their past FDA experience reviewing connected health products. Our team will also host a booth to discuss how outsourcing drug delivery device activities to our experts can save you time and money. Reach out to set up an appointment or stop by!

FORMER FDA REVIEWER CAROLYN DORGAN SHARES INSIDER TIPS FOR REGULATORY SUCCESS OF DRUG DELIVERY SYSTEMS FOR CELL AND GENE THERAPIES
Take advantage of a unique opportunity to hear directly from a drug delivery device specialist former FDA Reviewer / Infusion Devices team lead about how to achieve regulatory success in targeted delivery of cell and gene therapies. Carolyn Dorgan knows first-hand what works, what doesn’t, what will slow you down, and what will get you the approval you are seeking on the fastest pathway…and she will share this with you at PharmaEd Resources’ Combination Products Summit. Our team will also be there (booth # 1B) to discuss how you can cut costs and time to launch by outsourcing drug delivery device activities to our experts. Reach out to set up an appointment or stop by!
The complexities of combination product development and regulatory approval are ever-changing.
Advancements in science, technology and patient-care require continuous adjustments by the FDA, the EU MDA and other global regulatory agencies. This is why it is important to have a team of experts who are well- versed in current standards, practices and regulations on your team.
When a pharmaceutical company is working to bring a new, life-changing combination product to patients, Suttons Creek and its device and digital support platform specialists bridge the gap between pharma’s drug expertise and the highly specific, varying and intricate world of combination product regulations.
Our vantage point of experience gives our clients peace of mind, knowing that they are making decisions based on proven success.
FULL-SERVICE, CRADLE-TO-LAUNCH SUPPORT
Our team of medical device experts provides support through your entire product life cycle, from project inception to commercialization and post-launch activities. We integrate into your team and execute from day one but can also jump in at any stage in crisis.
ROBUST QUALITY ASSURANCE
We have a combined 650+ years of medical device and combination product experience, and we have worked on over 120 products, encompassing a wide variety of drug delivery systems and supporting digital software, for over 70 pharma clients. Our specialists have the experience to have “seen it before” and the know-how to navigate around all of the potential pitfalls of the process. Auto Injectors, Pre-Filled Syringes, On-Body Devices, Software as a Medical Device (SaMD) and more, we can help you develop it.
A PROVEN SYSTEM
We have an established track record for reducing execution risk and development timelines for our clients, while increasing efficiencies and product consistency, quality, reliability, and robustness. From these successful ventures, we have created proprietary systems to set your combination product up for a quick and painless global regulatory process. Whatever medical device you’re working on, there’s a great chance we’ve helped a pharmaceutical company like yours succeed in that space.
ADAPTIVE AND CUSTOMIZED APPROACH
Our consultants are specialists who work as an extension of your team to first identify what your drug delivery system, software, or quality needs are, and then tailor our approach to ensure your desired outcomes and timelines are met. We work with an adaptive, agile and customized systems approach that solves your specific problems without impeding on your existing medicine and molecule processes already in place.
