Combination Product Industry News & Guidance
Sharing device-related information and wisdom
that will help you succeed
Did you play the game on LinkedIn? Time to check your answers…
August 3, 2023 LinkedIn Post
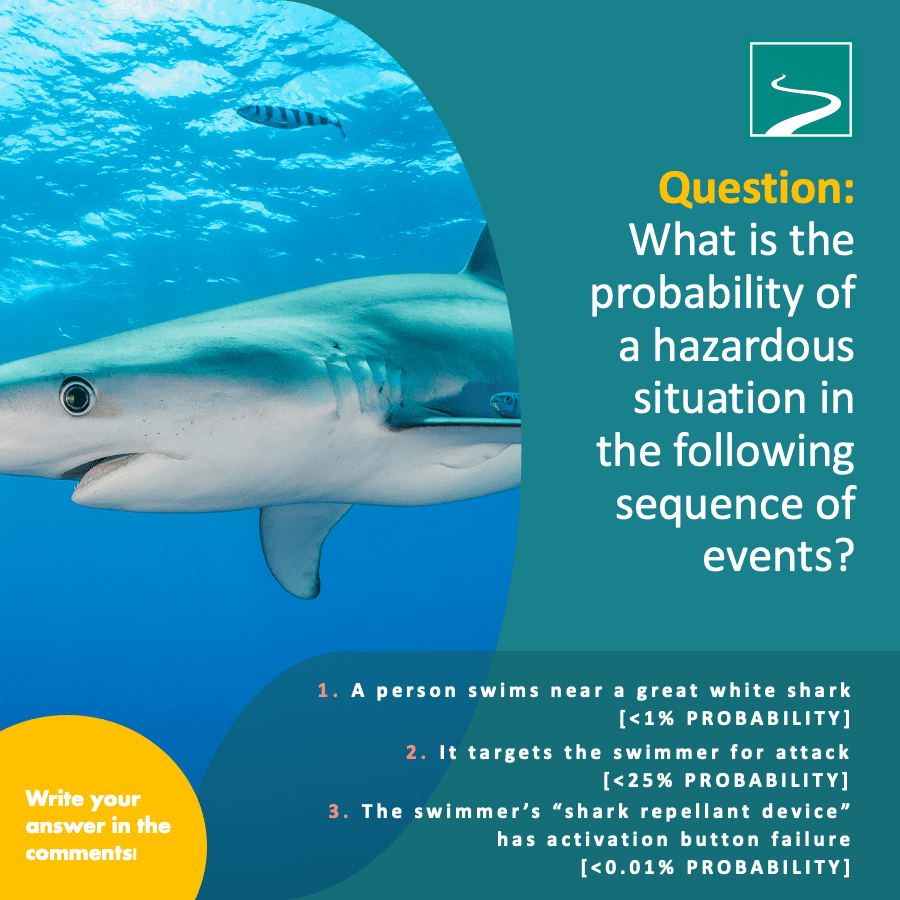
The Question...
What is the probability of a hazardous situation in the following sequence of events?
- A person swims near a great white shark [<1% probability]
- The great white targets the swimmer for attack [<25% probability]
- The person’s “shark repellant device” has activation button failure [<0.01% probability]
The Answer...
The probability of a hazardous situation is <0.000025. To get this result, we simply multiply the three events within the sequence. So, while swimming near a great white shark and having a 25% chance that it will target you for attack, the likelihood of your (make-believe) shark repellant device having activation button failure is so low that it significantly reduces the risk of actually being in a hazardous situation. We use calculations like this in risk management assessments of our clients’ (real) combination products.
To read more on the subject, check out our article Risk Management Assessment Calculations for Combination Products.
July 12, 2023 LinkedIn Post

The Question...
What is missing? Below is a list of documents/reports typically included in a combination product’s Design History File (DHF), but we have left some blanks for you to fill in…
- Design and Development Plan
- Design Inputs
- ________________
- Design Reviews
- Design Verification and Validation
- ________________
- ________________
- Risk Management File
- ________________
- Clinical Data
- Manufacturing and Quality
- Device Master Record / Master Batch Record
The Answer...
The missing documents are Design Outputs, Change Control, Design Transfer, and Usability Engineering File. Once you gain clarity on the regulatory requirements of your particular combination product, it is a good idea for a knowledgeable expert to create a Design History File (DHF) Index. Doing so at the outset of the project will ensure the necessary information in captured and documented within the final DHF.
To read more on the subject, check out our article Creating A Design History File (DHF) That Gets Approval
June 28, 2023 LinkedIn Post
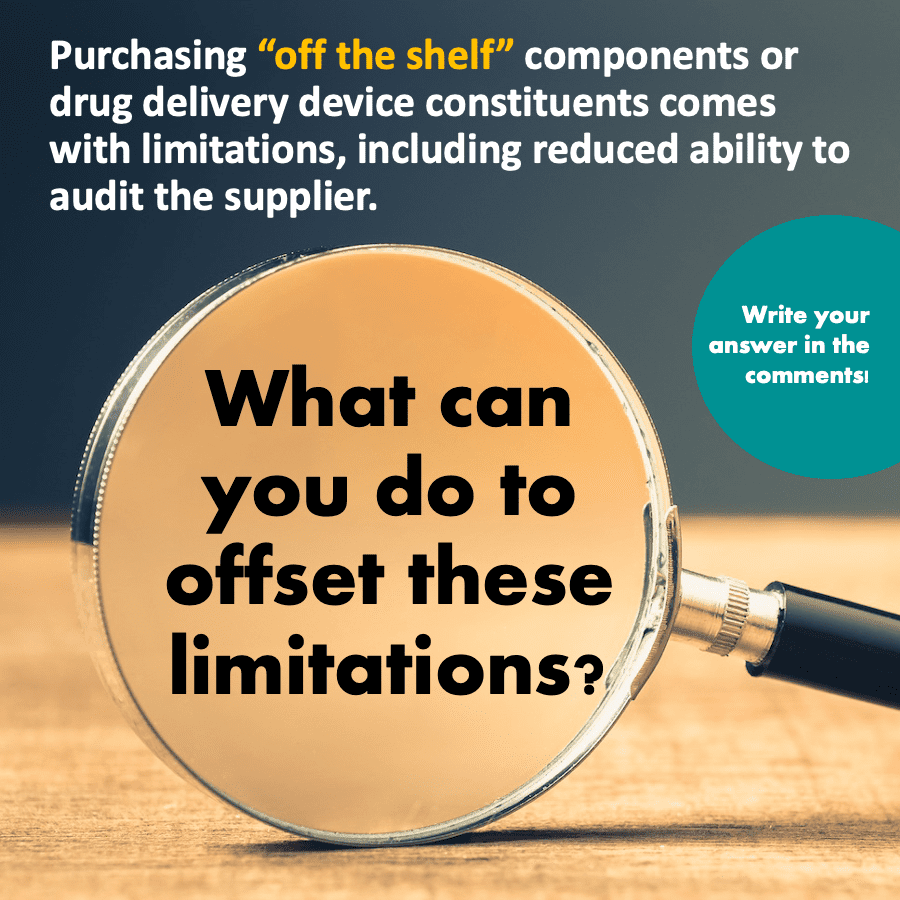
The Question...
Purchasing “off the shelf” components or drug delivery device constituents comes with
limitations, including reduced ability to audit the supplier. What can you do to offset these limitations?
The Answer...
To offset reduced ability to audit as a result of purchasing “off the shelf” components or drug delivery device constituents, industry best practices tells us it is a good idea to do all of the following:
- Conduct further testing to supplement incomplete information, including
usability and incoming release testing. - Have an agreed-upon change notification process and ALSO monitor for changes
internally. - Consider safety stock inventory.
- Conduct due diligence and quality monitoring in lieu of audits.
These actions are also good strategies to offset other “off the shelf” product quality management limitations, such as inadequate visibility to upcoming changes, quality concerns or supply issues, and inability to get access to complete information and documentation “off the shelf” items, particularly if you are using the item in a different way for which it was originally designed by the manufacturer.
Want more tips on effective quality management of your suppliers? Read our article “Are You Managing Your Combination Product Supplier Relationships Well Enough?”
June 7, 2023 LinkedIn Post

The Question...
How would you rank these three criteria for drug delivery device vendor/supplier selection in
your own decision–making process? (As we know, sometimes weighting decision–making criteria
is the only way to find a clear winner.)
A. Quality Management System
B. Technical Support / Communication / Project Management
C. Level/Quality of shared Design History File documentation
The Answer...
There’s no right–in–every–situation answer here. What is important is the process that you go
through to identify the risk and the potential costs or additional resources required
if your supplier falls short in any of these areas.
For more of Suttons Creek’s recommended selection criteria, read our article “Nine Ways To
Make Sure You Are Selecting The Right Device Constituent Supplier”
May 24, 2023 LinkedIn Post

The Question...
When developing a combination product, your drug and device teams need to work together. However, drug and device teams do not always speak the same language, even though they are performing very similar functions. Can you fill in the blanks?
Drug Development (QbD) calls it a Master Project Plan, but Device Development (Design Controls) calls it a ________________.
Device Development (Design Controls) calls it Human Factors, but Drug Development (QbD) calls it ________________.
Drug Development (QbD) calls it Characterization / Stability, but Device Development (Design Controls) calls it ________________.
Device Development (Design Controls) calls it a Design History File, but Drug Development (QbD) calls it a ________________.
The Answer...
Drug Development (QbD) calls it a Master Project Plan, but Device Development (Design Controls) calls it a Design & Development Plan.
Device Development (Design Controls) calls it Human Factors, but Drug Development (QbD) calls it Guidance on Medication Errors.
Drug Development (QbD) calls it Characterization / Stability, but Device Development (Design Controls) calls it Design Verification.
Device Development (Design Controls) calls it a Design History File, but Drug Development (QbD) calls it a Product Dossier & Development Report.
Here are a few more similar functions that are called something different in Drug Development (QbD) v. Device Development (Design Controls):
Master Project Plan v. Design & Development Plan
Quality Target Product Profile v. User Needs & Design Inputs
CQA, Control Strategy v. Design Outputs
CMC Stage Gate Reviews v. Design Reviews
Characterization / Stability v. Design Verification
Clinical Studies v. Design Validation
Guidance on Medication Errors v. Human Factors
Technology Transfer v. Design Transfer
Product Dossier & Dev. Reports v. Design History File
Master Batch Record v. Device Master Record
Risk Management ICH Q9 v. Risk Management ISO 14971
Change Control v. Design Change Control

